
He won the 1906 Nobel prize for the discovery of electrons.

He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, a British physicist, conducted the cathode ray experiment. In addition, the charge to mass ratio of ionized particles was found to be different and depended on the type of gasses.Previously, atoms were known to be indivisible, but in 1897, J. Thomson measured the deflection of the beam using a ruler etched on the end of. You can see a simulation of this glow on the far right of the applet diagram, as shown in Figure 2. It needed a high voltage of the order of 30,000 volts. In Thomson’s experiment, a fluorescent material was coated on the end of the tube to produce a glowing dot where the cathode rays hit. It is because the canal rays consist of positive ions formed as a result of gas ionization. The cathode, in the discharge tubes used by Sir Thomson, was cold cathode. The canal rays depend on the nature or type of gas present inside the glass tube and are different from cathode rays. In addition, the canal rays are considered to be the nucleus of the gas which has differentiated properties compared to cathode rays. Where E is the energy gained, q is the charge of the. When a high external voltage source is applied to the glass tube via electrodes, it ionizes the gas present inside the tube where the positive ions constitute the origin of the canal ray. The energy gained by the electrons in a cathode ray tube can be calculated using the formula: E qV. The prediction for this lab was that if the particle begins with a higher voltage, then thedisplacement would be smaller and an equation of Deflection((Vd)(L)(.

Increase the external voltage source to several thousand volts, which results in the emission of faint luminous rays emerging from the cathode electrode holes. radius of this trajectory, R, to the strength of the B-field and the accelerating voltage (anode voltage).

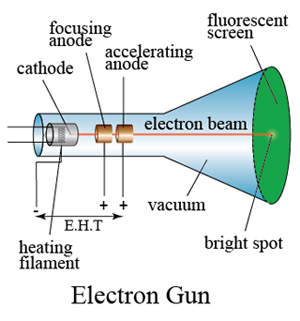
This leads to the completion of the circuit and apparatus setup. High voltage passing through the electrode ionizes the air and makes it conduct the electricity like a conductor.This is the basis of the important experiments carried out by J J Thomson and others. In the experiment of measuring the charge-to-mass ratio of electron with cathode-ray tube, the velocity component of electron is generated by a constant. This cathode ray can be focused and deflected and can carry small currents. Connect the two electrodes (anode and cathode) to a high external voltage source. However, if there is a small hole in the anode, some electrons will pass through, forming a beam of electrons that came from the cathode or a cathode ray.Since electrons are repelled by the negative electrode, the cathode is the source of cathode rays inside a vacuum environment. 1 Im looking for a suitable high voltage source for the electron gun of a cathode ray tube (CRT.) Slightly simplified the schematic of a CRT looks like: This eBay listing seems to offer something that might be suitable: mains feed, adjustable high voltage (DC) output, fairly detailed description/manual. the table in the A.C measurement voltage and sensitivity determinant value experiment calculate the Vmax. To maintain a low pressure inside the glass tube, evacuate the air through the vacuum pump. y 1 2 a y t 2 From the capacitor voltage U A and the distance of the capacitor plates d the electric force F of the field can be determined. The cathode is a negative electrode, Anode is the positive electrode. Some Measurements Using Cathode Ray Tube (CRT).To do this, we create a uniform electric field in the region between the deflecting plates by applying a voltage, Vd, across the plates, as seen in Figure 2. The glass tube pressure is minimized through vacuum pumps or evacuating the air. v acc m (3)Once electrons leave the electron gun, we will use electric fields to steer the electron beam.A high voltage source (external) is connected to the metal electrodes.A Cathode ray tube made of glass with two metal electrodes ( anode and cathode) on each differentiated side.


 0 kommentar(er)
0 kommentar(er)
